scanpy是单细胞数据分析的python工具包,将数据以Anndata对象的格式进行储存。如下将学习Anndata对象操作以及scanpy分析的基础用法。
1
2
3
|
import numpy as np
import pandas as pd
import anndata as ad
|
1、数据导入#
1
2
3
4
5
6
7
8
9
10
|
# !wget http://cf.10xgenomics.com/samples/cell-exp/1.1.0/pbmc3k/pbmc3k_filtered_gene_bc_matrices.tar.gz
# tar -xzf pbmc3k_filtered_gene_bc_matrices.tar.gz
adata = sc.read_10x_mtx(
'filtered_gene_bc_matrices/hg19/', # the directory with the `.mtx` file
var_names='gene_symbols') # use gene symbols for the variable names (variables-axis index)
adata
# AnnData object with n_obs × n_vars = 2700 × 32738
# var: 'gene_ids'
|
1
2
3
4
5
6
7
8
9
10
11
12
13
14
|
## 同样是上述示例数据,先用R包Seurat处理,储存结果
library(Seurat)
pbmc.data = Read10X(data.dir = "filtered_gene_bc_matrices/hg19/")
pbmc = CreateSeuratObject(counts = pbmc.data, project = "pbmc3k")
pbmc = NormalizeData(pbmc) %>%
FindVariableFeatures()
pbmc = pbmc %>%
ScaleData() %>%
RunPCA() %>%
RunUMAP(dims = 1:30) %>%
FindNeighbors(dims = 1:30) %>%
FindClusters(resolution = 0.1)
head(pbmc@meta.data)
saveRDS(pbmc, file = "pbmc.rds")
|
1
2
3
4
5
6
7
8
9
|
## 准备Python环境
import scanpy as sc
import numpy as np
import anndata2ri
# Activate the anndata2ri conversion between SingleCellExperiment and AnnData
anndata2ri.activate()
#Loading the rpy2 extension enables cell magic to be used
#This runs R code in jupyter notebook cells
%load_ext rpy2.ipython
|
1
2
3
4
5
|
## 读入对象
%%R
library(Seurat)
pbmc = readRDS("pbmc.rds")
pbmc
|
1
2
3
4
5
|
## 转换对象
%%R -o pbmc_sce
#convert the Seurat object to a SingleCellExperiment object
pbmc_sce <- as.SingleCellExperiment(pbmc)
pbmc_sce
|
1
2
3
4
5
6
|
## 查看转换后的anndata对象
pbmc_sce
# AnnData object with n_obs × n_vars = 2700 × 32738
# obs: 'orig.ident', 'nCount_RNA', 'nFeature_RNA', 'RNA_snn_res.0.1', 'seurat_clusters', 'ident'
# obsm: 'X_pca', 'X_umap'
# layers: 'logcounts'
|
2、Anndata结构#
https://anndata-tutorials.readthedocs.io/en/latest/getting-started.html
以上述1.2的pbmc_sce对象为例学习anndata结构组成
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
|
adata = pbmc_sce
# (1) 矩阵
# !注意:行是细胞,列是基因。与Seurat读取结果相反!
adata.obs_names #行名(细胞名)
adata.var_names #列名(基因名)
## 原始信息以稀疏矩阵形式储存
adata.X
print(adata.X)
adata.X.todense()
adata.layers["log_transformed"] = np.log1p(adata.X)
# adata.layers["counts"] = adata.X.copy()
adata.to_df(layer="log_transformed")
adata.layers["X"] = adata.X
adata.to_df(layer="X")
# (2) metadata,按pd.Dataframe格式储存
adata.obs # 细胞的metadata(常用)
adata.var # 基因的metadata
# (3) 降维数据:多是数组格式,按字典逻辑储存
adata.obsm
adata.obsm.keys
adata.obsm["X_umap"]
# (4) subset子集
adata[["Cell_1", "Cell_10"], ["Gene_5", "Gene_1900"]]
adata[adata.obs.cell_type == "B"]
|
3、scanpy基础分析#
https://scanpy-tutorials.readthedocs.io/en/latest/pbmc3k.html
以上述1.1的原始读取结果adata对象为例
1
2
3
4
5
6
7
8
9
10
11
|
sc.pp.filter_cells(adata, min_genes=200)
sc.pp.filter_genes(adata, min_cells=3)
adata.var['mt'] = adata.var_names.str.startswith('MT-')
sc.pp.calculate_qc_metrics(adata, qc_vars=['mt'],
percent_top=None, log1p=False, inplace=True)
# sc.pl.violin(adata,
# ['n_genes_by_counts', 'total_counts', 'pct_counts_mt'],
# jitter=0.4, multi_panel=True)
adata = adata[adata.obs.n_genes_by_counts < 2500, :]
adata = adata[adata.obs.pct_counts_mt < 5, :]
|
1
2
3
4
|
sc.pp.normalize_total(adata, target_sum=1e4)
sc.pp.log1p(adata)
sc.pp.scale(adata, max_value=10)
sc.pp.regress_out(adata, ['total_counts', 'pct_counts_mt'])
|
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
|
## 高变基因
sc.pp.highly_variable_genes(adata)
adata.var
## PCA降维
sc.tl.pca(adata)
adata.obsm["X_pca"].shape
sc.pl.pca(adata) # 默认按X_pca的降维结果可视化
sc.pl.pca(adata, color='CST3')
## UMAP降维
sc.pp.neighbors(adata, n_neighbors=10, n_pcs=40)
sc.tl.umap(adata)
sc.pl.umap(adata)
## 分群
sc.tl.leiden(adata, resolution=0.5,key_added="cluster")
adata.obs
sc.pl.umap(adata, color=['cluster'])
|
1
|
adata.write("your_data.h5ad")
|
2024-12-15
Loompy 是一个用于管理、存储和处理大规模单细胞基因表达数据的 Python 库,可以与Scanpy,Seurat等兼容。其中Loom 格式专门用于高效地存储稀疏矩阵及其相关的元数据。
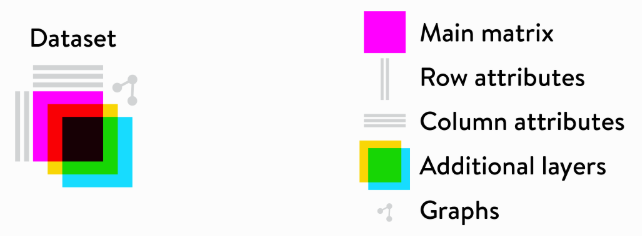
下面学习其基本用法—
1
2
3
|
import scanpy as sc
import loompy
import numpy as np
|
1
2
3
4
5
6
7
8
9
|
adata = sc.read_h5ad("demo_adata.h5ad")
# 添加一个Layer
adata.layers["count"] = adata.X.copy()
# 选择需要保存的row/col metadata
adata.var = adata.var[["feature_id"]]
adata.obs = adata.obs[["raw_sum"]]
# 最后保存:
adata.write_loom("./demo_adata.loom")
|
与anndata的两点区别:
- loom储存的矩阵,row代表基因,column代表细胞。这anndata正好相反
- loom储存更加高效,占用硬盘空间相比h5df格式,可显著节约内存。
直接读取为anndata对象
1
2
|
adata = sc.read_loom("./demo_adata.loom")
adata
|
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
|
# (1) 两种方式读取,后者适用于在交互式环境操作
with loompy.connect("filename.loom") as ds:
# do something with the connection object ds
ds = loompy.connect("filename.loom")
# do something with the connection object ds
ds.close()
# (2) Layers
ds.layers.keys()
# ['', 'count']
ds[:4,:4]
ds["count"][:4,:4]
# (3) row/col attribute
ds.ra.keys()
# ['ensembl_id', 'var_names']
ds.ca.keys()
# ['n_counts', 'obs_names']
ds[:, ds.ca.n_counts < 3000].shape
# (4) map func
ds.map([np.mean, np.std], axis=1)
# (5) scan: 对每个batch细胞分别处理
for (ix, selection, view) in ds.scan(axis=1, batch_size=1024):
print(view.shape)
break
ds.close()
|
在使用过程中,遇到numpy>2.0的版本冲突问题。根据提示,修改源码即可。
